Net Ionic Equation for Acid Base Reaction
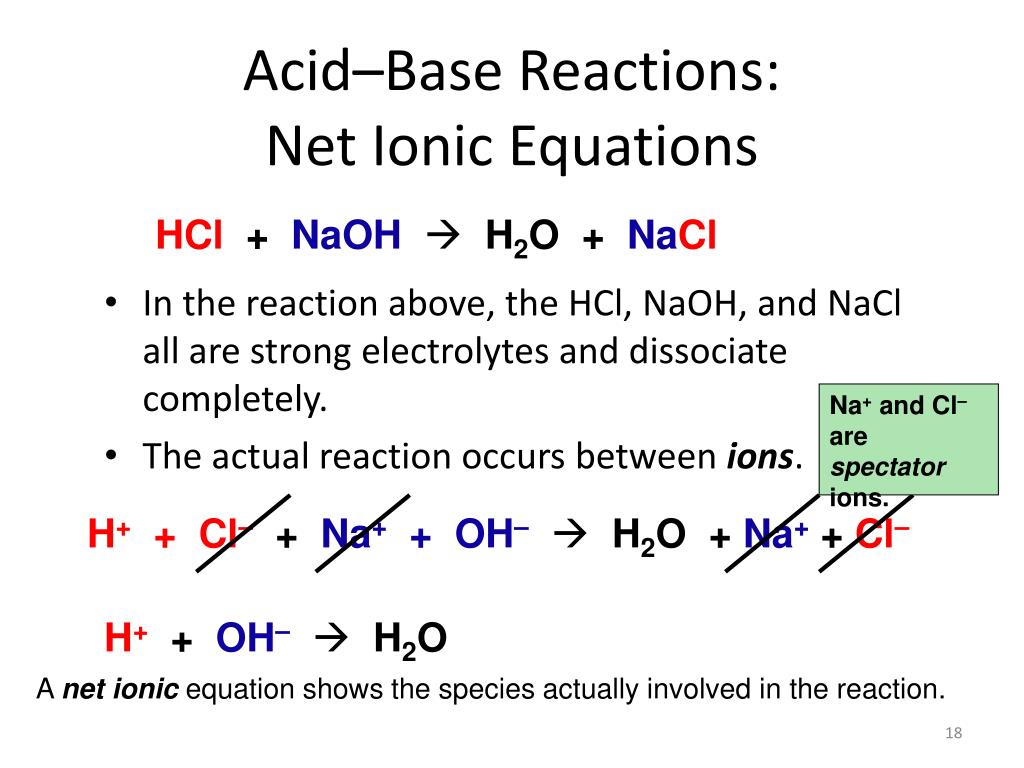
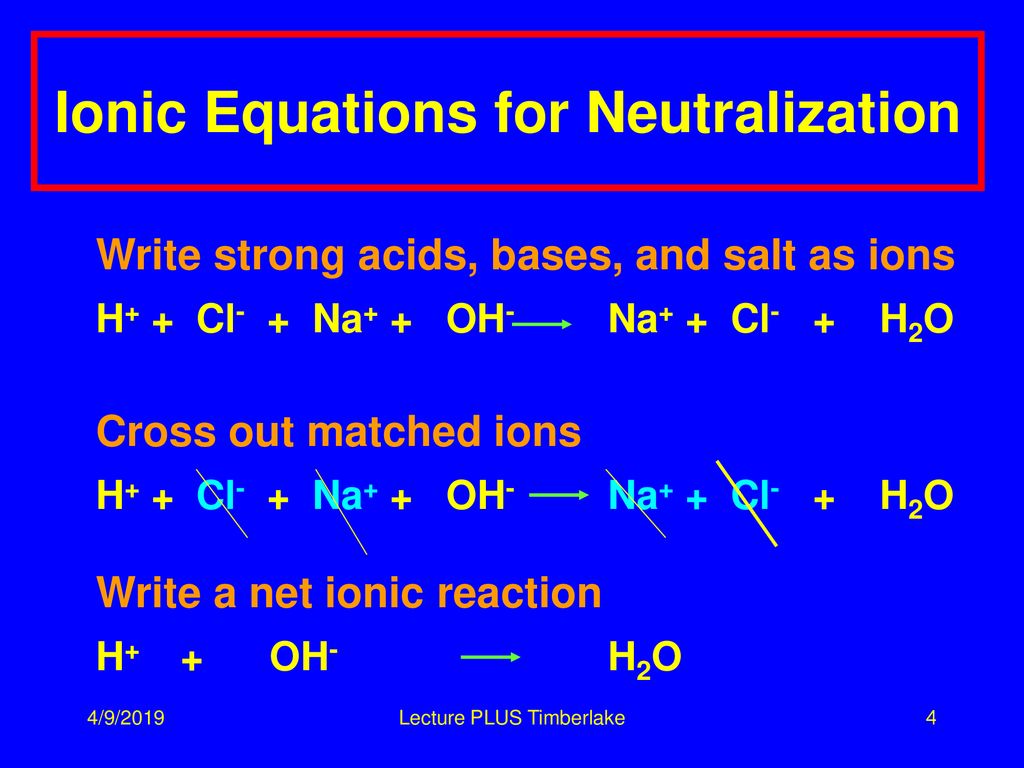
All neutralization reactions of a strong acid with a strong base simplify to the net ionic reaction of hydrogen ion combining with hydroxide ion to produce water. Strong bases are considered strong electrolytes and will dissociate completely.
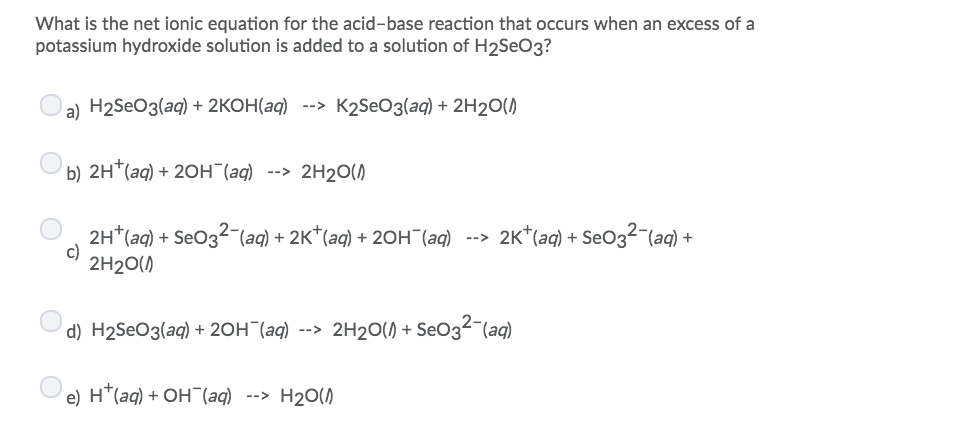
Solved What Is The Net Ionic Equation For The Acid Base Chegg Com
So when you add them together you end up with NH4Cl then the water is in the solution that you have there.

. What if the acid is a diprotic acid such as sulfuric acid. The difference is simply the. Net Ionic Equation For Perchloric Acid And Potassium Hydroxide.
It is the OH- hydroxyl ion which makes NaOH a base. The reaction of Nitric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base. Weak acids only dissociate partially and are not considered to split apart into.
Correct answer to the question Write a net ionic equation for the neutralization reaction of acid with a hydroxide base. Here carbonic acid is a weak acid and potassium hydroxide is a strong base. The difference is simply the presence of an extra water molecule as a product.
February 20 2022 by czech. Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chloride. In other words the net ionic equation applies to reactions that are strong.
The equation can be written in terms of ions as. Get the full course at. View the full answer.
HClO₄aq NaOHaq. The reaction of chlorous acid weak acid and sodium hydroxide strong base is written as. The net ionic equation for this reaction is.
One may also ask is HCl a strong acid. This means that we will split them apart in the net ionic equation. Two types of Chemical reactions includes.
The ions which are present on both the sides of the equation are sodium ions and hence are not involved in net ionic equation. Because the salts are soluble in both cases the net ionic reaction is just H aq OH aq H 2 Oℓ. What is the net ionic reaction for an acid base neutralization reaction.
Haq OHaq H₂Ol Complete and balance the acid-base reaction. Lets write the molecular total ionic and net ionic equations for an ACID BASE neutralization reaction. C H aq OH- aq H2O l When an acid H2SO3 reacts wi.
NaOH is a base because when dissolved in water it dissociates into Na and OH- ions. The reaction of Sodium hydroxide and Acetic acid also called Ethanoic acid represents a net ionic equation involving a strong base and a weak acid. We never disclose personal How To Write Net Ionic Equations For Acid Base Reactions information and encourage students to upload additional files to the profile to ensure the efficient work of the writer in the beginning.
Hence the net ionic equation is. The balanced molecular equation now involves a. NH4NO2 ammonium nitrite ionic.
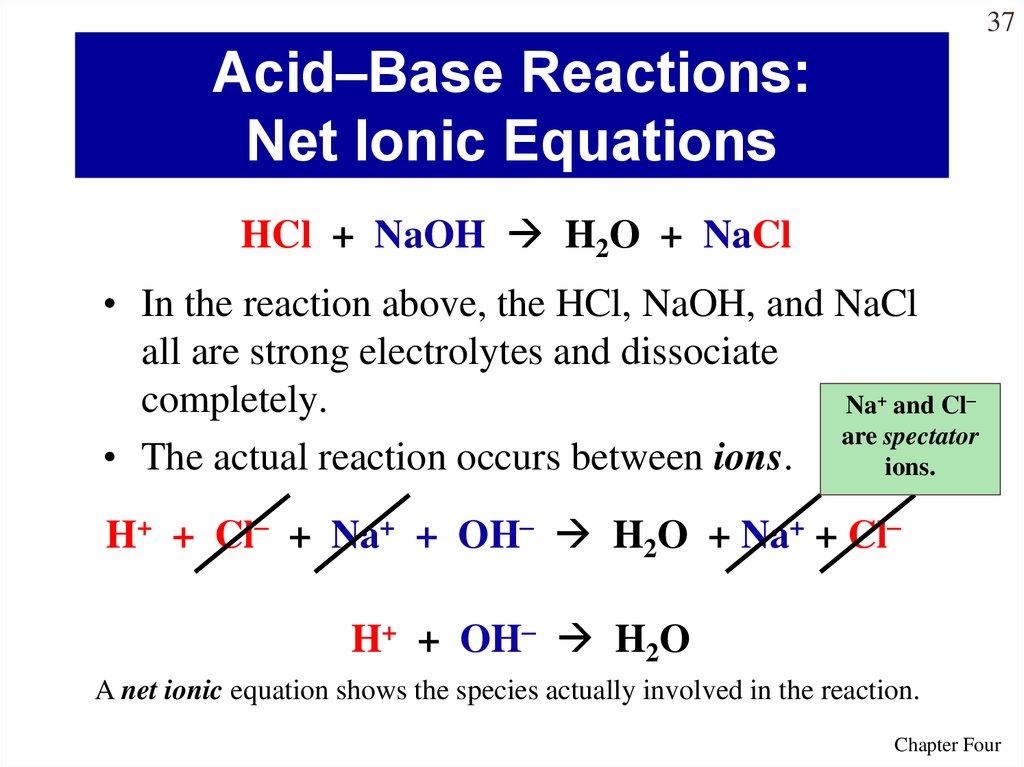
So with a strong acid and strong base the net ion equation is always H plus OH- yields H2O. The net ionic equation is a chemical equation for a reaction that lists only those species participating in the reaction. A 2H aq S032- aq 2Na aq 2OH aq 2Na aq S0 2 aq 2H2O 1 O b.
When a hydrochloric acid solution and a potassium hydroxide solution are combined an acid-base reaction occurs. Write a balanced net ionic equation for the reaction. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
What is the net ionic reaction for an acid base neutralization reaction. Lets see if writing the net ionic equation can help answer our question. This means that we will split them apart in the net ionic equation.
A chemical reaction shows us how substances interact which brings about changes in the composition of the reactants. The net ionic equation is commonly used in acid-base neutralization reactions double displacement reactions and redox reactions. Strong acids and strong bases are considered strong electrolytes and will dissociate completely.
So the Na and the Cl - are spectator ions. Think about this question. 2-Consider the reaction when aqueous solutions of lead nitrate and aluminum sulfate are combined.
Acid as proton donor HCl HCl aq - H aq Cl-Base as OH donor NaOH NaOHaq - Na OH-HCl NaOH Total Equation HClaq NaOHaq - H 2 Ol NaClaq Dissociation HCl g - H aq Cl-aq NaOH - Na aq OH-aq Neutralization spectator ions H 3 O Cl- Na OH-- 2 H 2 O Na Cl-Net Ionic. A strong acid is an acid which is completely ionized in an aqueous solution. Net Ionic Equation Definition.
HNO 3 NaOH NaNO 3 H 2 O is a neutralization reaction also a double displacement. The net ionic equation for the acid-base reaction of hydrochloric acid with phosphine is PH3aq Haq PH₄ aq What is a Chemical reaction. The net ionic equation.
So in number two say if we have HCl and Ammonia NH3. Definition of an acidbase reaction. Because the salts are soluble in both cases the net ionic reaction is just H aq OH aq H 2O ℓ.
Is NaOH an acid or base. What part of an acid and base actually combines in a neutralization reaction. So with a strong acid and strong base the net ion equation is always H plus OH- yields H2O.
Ammonia is a weak base. We can be How To Write Net Ionic Equations For Acid Base Reactions considered a reliable service for a number of reasons that actually make sense. But before we dive in.
What is the net ionic equation for the acid-base reaction that occurs when an excess of a sodium hydroxide solution is added to a solution of H2SO3.
Ppt Solubility And Ionic Equations Powerpoint Presentation Free Download Id 3105358

Strong Acid Strong Base Reactions Video Khan Academy

Weak Acid Strong Base Reactions Video Khan Academy
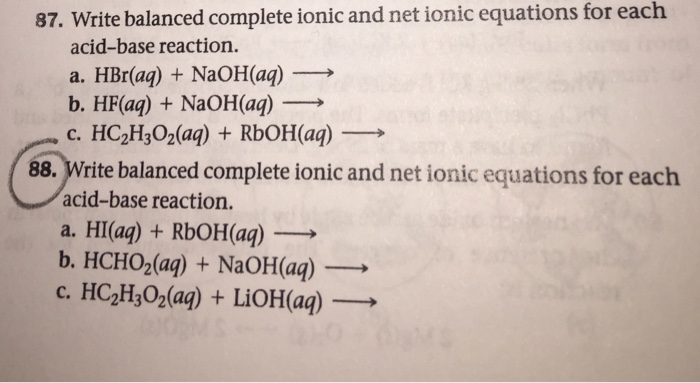
Solved Write Balanced Complete Ionic And Net Ionic Equations Chegg Com
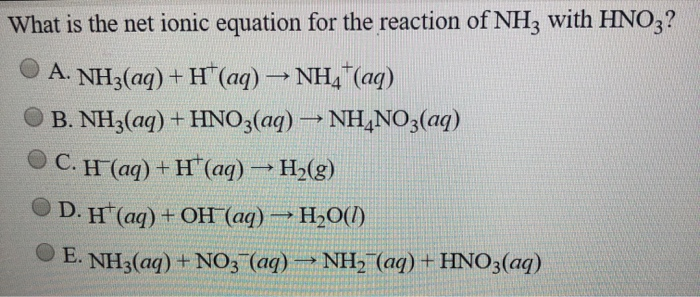
Solved What Is The Net Ionic Equation For The Acid Base Chegg Com

Tips For Acid Base Net Ionic Equations Concept Chemistry Video By Brightstorm

Chapter 10 Reactions In Aqueous Solutions I Acids Bases Salts Ppt Video Online Download
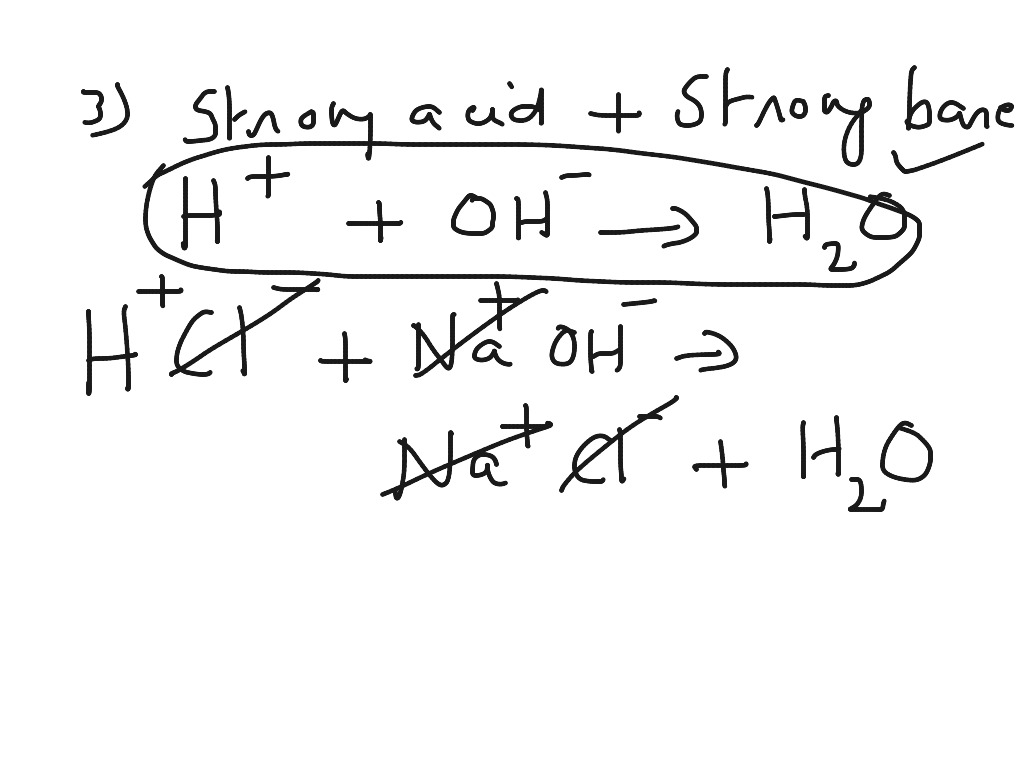
Net Ionic Equation For Acid Base Reaction Science Showme

Acid Base Neutralization Reactions Net Ionic Equations Chemistry Youtube
Aqueous Solutions Of Electrolytes Prezentaciya Onlajn
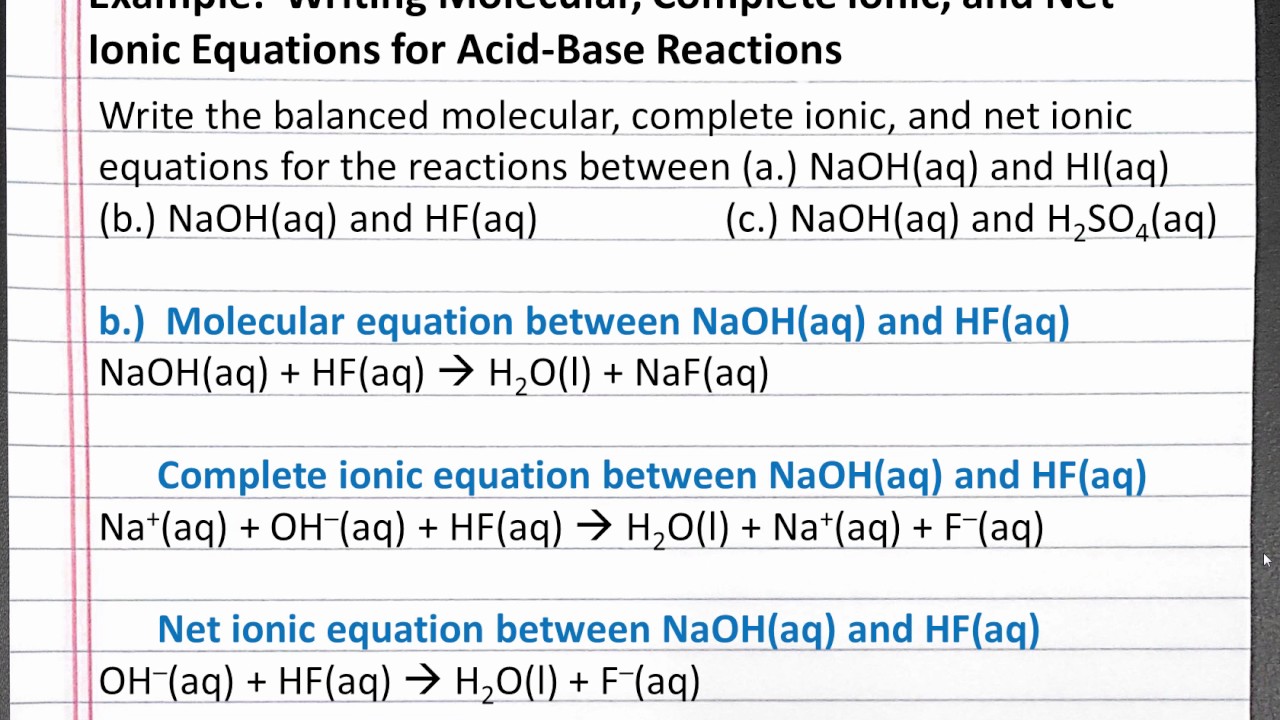
Chemistry 101 Writing Molecular Complete Ionic And Net Ionic Equations For Acid Base Reactions Youtube
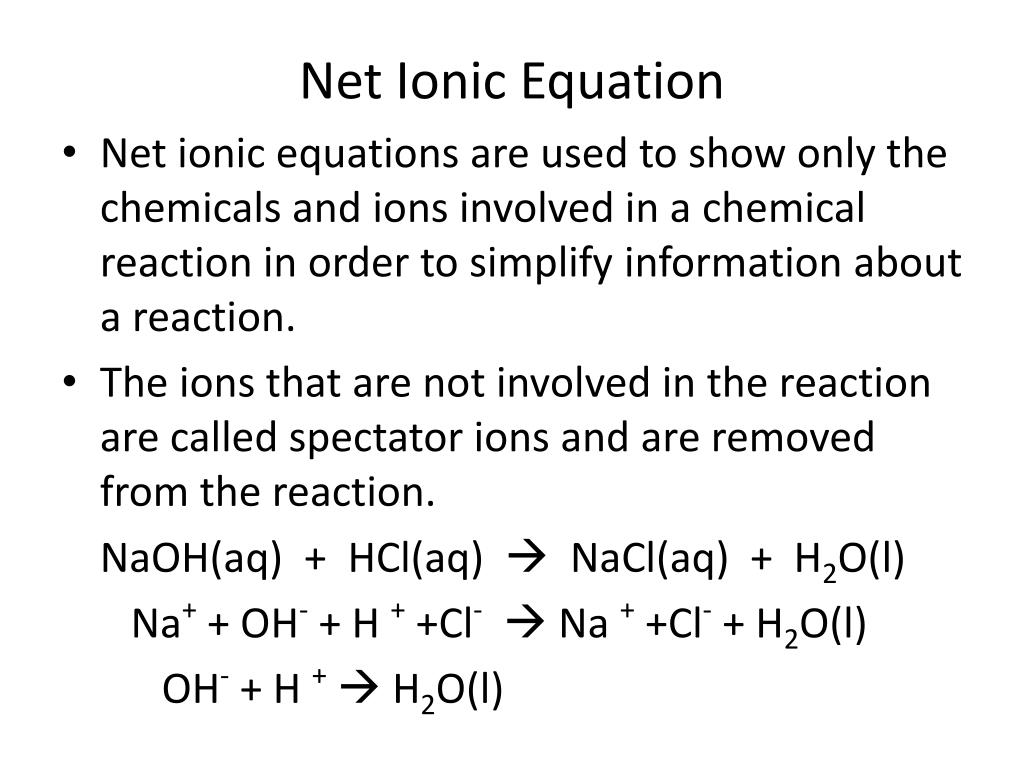
Ppt Net Ionic Equation Powerpoint Presentation Free Download Id 3561134

Net Ionic Equations With Acids Bases Ap Chem Youtube

Question Video The Net Ionic Equation For The Neutralization Reaction Between Ammonium Hydroxide And Hydrochloric Acid Nagwa

Net Ionic Equations Acid Base Ppt Gas Forming Youtube

Solved Complete And Balance Each Of The Following Acid Base Chegg Com
Chapter 3 4 Acids And Bases Ppt Download

Strong Acid Base Titrations Chapter 17 Neutralization Reactions Review Generally When Solutions Of An Acid And A Base Are Combined The Products Are Ppt Download

Tips For Acid Base Net Ionic Equations Concept Chemistry Video By Brightstorm
Comments
Post a Comment